PRODUCTOS SANITARIOS


.
REGLAMENTO MDR (EU) 2017/745
REGULATION MDR – ingles
entrada en vigor: 26 mayo 2017
fecha aplicación: 26 mayo 2021
«legacy» IIb implant y III 31 dic 2027
«legacy» IIa y I con ON 31 dic 2028
s/reglamento 2023/607
sin fin periodo distribución
.
PRODUCTOS SANITARIOS IVD


.
REGLAMENTO IVDR (EU) 2017/746
REGULATION IVDR – ingles
entrada en vigor: 26 mayo 2017
fecha aplicación: 26 mayo 2022
«legacy» D 31 Dic 2027
«legacy» C 31 Dic 2028
«legacy» B y As 31 Dic 2029
s/modificación reglamento 2024/
sin fin periodo distribución
.
PROXIMA FORMACIÓN

Formación «2405 – IMPORTADORES Y DISTRIBUIDORES DE PROD.SANIT. según REQUISITOS MDR / IVDR y RD» – 18 Abr 2024 9h-14h
Ahora que nos acercamos al 26 de mayo de 2024 se vuelven mas relevantes los reglamentos MDR/IVDR y nuevo Real Decreto 192/2023 (RD IVD en borrador) que incrementan los requisitos a los importadores y distribuidores involucrados en el suministro, instalación, formación y mantenimiento de productos sanitarios. Ahora estamos en un periodo transitorio en el que importadores y distribuidores deben revisar el cumplimiento por parte del fabricante de los productos para su introducción en el mercado y liberación por el importador y comercialización por los distribuidores.
En esta formación, revisaremos las obligaciones de los importadores y distribuidores así como el impacto de los requisitos reglamentarios en toda la cadena de suministro. Tenemos la posibilidad de realizar mas actividades antes consideradas exclusivas de fabricante como reembalado y traducción de IFU según art.16 pero con requisito de sistema de calidad ISO 13485 certificado, también el registro del UDI-DI, el nuevo Registro de Comercialización de la AEMPS, …
No te pierdas esta jornada donde contaremos las novedades relativas a estas actividades según los nuevos reglamentos para estos periodos transitorios y como verificar el cumplimiento.
Fecha: 18 Abril 2024, 9h a 14h en webinar en directo y 25h en teleformación
Esperamos verte ahi … ![]()

#ExpoÓptica24 y #ExpoAudio24 12-14 Abril 2024 @Expooptica la feria de la optica y la audiologia española
ExpoÓptica, Salón Internacional de Óptica, Optometría y Audiología, es la feria profesional de referencia del sector y punto de encuentro comercial para todos sus agentes en España y Portugal. Se presentan las marcas nacionales e internacionales de prestigio, que incluyen desde los últimos avances en el mundo de la óptica, la optometría y la audiología. Al ser las monturas y lentes ópticas de la clase I ahora ya todas con MDR, las lentes de contacto y audífonos aun en periodo transitorio pero muchos ya con marcado CE con MDR.
El Salón se celebra en paralelo a OPTOM, Congreso Internacional de Optometría, Contactología y Óptica Oftálmica, organizado por el Consejo General de Colegio de Ópticos-Optometristas.![]()

Exito de #EXPODENTAL2024 del 14 a 26 marzo 2022 Madrid – @ExpoDental_ by @FENIN_es
La feria de referencia a nivel internacional en España del sector dental español y europeo junto con la IDS de Colonia. Cada dos años, reúne en Madrid a empresas, asociaciones, centros de formación y universidades para mostrar a todos los profesionales de la industria las últimas novedades tecnológicas y tendencias, en un entorno cómodo, accesible e innovador tanto para visitantes como para expositores.
#EXPODENTAL2024 del 14 a 26 marzo 2022 Madrid – @ExpoDental_ by @FENIN_es
La feria de referencia a nivel internacional en España del sector dental español y europeo junto con la IDS de Colonia. Cada dos años, reúne en Madrid a empresas, asociaciones, centros de formación y universidades para mostrar a todos los profesionales de la industria las últimas novedades tecnológicas y tendencias, en un entorno cómodo, accesible e innovador tanto para visitantes como para expositores.

Team-NB publica el position paper «recommendations on the classification of devices intended to detect the presence or the exposure to SARS-CoV-2» cambio clase D a clase C
La Asociación Europea de Organismos Notificados de Productos Sanitarios TEAM-NB publica este nuevo position paper en el que indica que está de acuerdo en que la reclasificación de la clase D a una clase de riesgo inferior para el SARS-CoV-2 no pandémico es apropiada al haber declarado la OMS en fecha 5 desmayo de 2023 que el SARS-COV-2 (COVID 19) ya no era un PHEIC (public health emergency of international concern) y pasaba a fase endémica.
Teniendo en cuenta el riesgo potencial restante para las poblaciones vulnerables (en el peor de los casos) y los limitados datos disponibles para la fase pospandémica, se recomendaría una reclasificación a la clase C: esto garantizaría la aplicación de unos requisitos más estrictos en materia de PMS y evaluación del rendimiento/pruebas clínicas y una mejor protección de la seguridad de los pacientes.
También podría considerarse una nueva reclasificación a la clase B una vez que se disponga de más datos sobre la fase pospandémica, especialmente en lo que respecta al síndrome Covid persistente.
Descargue el documento del TEAM-NB aqui:![]()

Feria @CosmobeautyBCN 17 a 19 febrero 2024 para el sector de estética con productos de Anexo XVI MDR
La feria COSMOBEAUTY presenta las ultimas novedades del sector de la estética, entre los que hay diversos productos (laser depilación, IPL, radiofrecuencias, lentes contacto cosméticas, implantes mamarios, rellenos intradermicos, …) que están regulados como productos sanitarios por el reglamento MDR a partir de 22 de junio de 2023 y cuyo periodo transitorio para los legacy esta en cuenta atras. Incluye el Congreso Internacional de Estética y Spa (CIES), que se lleva a cabo durante los 3 días de evento, y que no incluye ninguna ponencia sobre el tema (la técnica del avestruz ….).![]()
nos vemos en CosmoBeauty …
2023 – Resumen Productos Sanitarios – Medical Devices Recap
Termina un año lleno de novedades y sorpresas y queremos desde aqui hacer un resumen del mismo:
– ENE – 37 ONs MDR y 8 ONs IVDR https://www.tecno-med.es/nuevo-on-mdr-0494/
– 16 FEB – Aprobada la extensión de periodos transitorios https://www.tecno-med.es/propuesta-modifi…dr-ivdr-aprobada/
– 20 MAR – Publicado Reglamento 2023/607 de modificación de plazos transitorios de MDR e IVDR https://www.tecno-med.es/reglamento-2023_…icacion-mdr-ivdr/
– 22 MAR – Publicado Real Decreto 192/2023 por el que se regulan los productos sanitarios entrando en vigor 23 marzo 2023 https://www.tecno-med.es/rd-ps-192_2023/
– 27 MAR – IMDRF International Medical Devices Regulators Forum en Bruselas https://www.tecno-med.es/imdrf-europe2023-final/
– 24 MAY – 1er Congreso SEDE Sociedad Española de Desinfección y Esterilización https://www.tecno-med.es/sede-mayo2023-completo-2/
– 26 MAY – 1er cumple aplicación IVDR y 2º cumple aplicación MDR https://www.tecno-med.es/2304-2-2/
– 20 JUN – Reglamento 2023/1194 que extiende los plazos de fin de periodos transitorios para productos Anexo XVI https://www.tecno-med.es/reglamento-exten…plazos-anexo-xvi/
– 22 JUN – Entrada en aplicación el reglamento MDR y RD 192/2023 a los productos de la lista de anexo XVI
– 18 JUL – MedtechEurope ( FENIN ) publica un modelo de «Manufacturer’s Declaration» a emitir por el fabricante https://www.tecno-med.es/manufacturer-declaration-medtech/
– 25 SET – IMDRF Berlin https://www.tecno-med.es/imdrf-europe2023berlin-2/
– 13-16 NOV – MEDICA https://www.tecno-med.es/medica2023-dia1/ https://www.tecno-med.es/medica2023-dia2/ https://www.tecno-med.es/medica2023-dia3/ https://www.tecno-med.es/medica2023-dia4/
– 22 NOV – Premios Tecnologia Sanitaria 2023 y Homenaje a Margarita Alfonsel https://www.tecno-med.es/homenaje-margarita-alfonsel/
– 30 NOV – Reunión EPSCO con tema de plazos, EUDAMED y en especial IVDR sobre la mesa https://www.tecno-med.es/epsco-30nov2023-2-2/
– 9 DIC – Aprobada la AIA Artificial Intelligence Act en el trilogo https://www.tecno-med.es/aprobada-la-aia-…as-enhorabuena-2/
– 16 DIC – Encuesta Comisión sobre estado MDR / IVDR https://www.tecno-med.es/com-survey-2023-12-2-2/ hasta 15 enero 2024
– 21 DIC – Estado proceso designación ONs https://www.tecno-med.es/estado-on-202312/
Como veis estamos totalmente enfrascados en la transición y en la cuenta atrás de 26 mayo 2024 donde sabremos que pasa con muchos de los legacy … y en mayo 2025 es cuando tenemos el fin para los clase D de IVDR …
Interesante el informe de MEDTECH sobre el sector en números
OS DESEAMOS FANTASTICO 2024 !!!
Team-NB publica el position paper «Medical Device Lifetime»
La Asociación Europea de Organismos Notificados de Productos Sanitarios TEAM-NB publica este nuevo position paper en la que nos da criterios para el establecimiento de la vida útil de productos en los que su análisis es difícil comeos software, los implantes, …
Felices Navidades !!! os deseamos desde @tecno_med

MEDICA @MEDICAtradefair 16 Nov 2023, resumen dia 4, ultimo día
16 nov 2023
Ya se termina el cuarto y último día de MEDICA,
– ayer nos fuimos todos puntuales (18h) con el anuncio de huelga de trenes pensando habría caos circulatorio … y no
– Ambiente de despedida en Aldstad (Café Madrid, …)
– vino caliente y bratwurst en Burgplatz mientras miramos el patinaje sobre hielo
read more…

MEDICA @MEDICAtradefair 15 Nov 2023, resumen dia 3
15 nov 2023
Ya se termina el tercer dia de MEDICA,
– Hoy tenemos un conclave regulatorio español Maria Alaez y Cristina Batlle dos grandes expertos en FENIN, cualquier duda aprovechad …
– se mantiene el alto nivel de visitantes, muchas reuniones y actividad en stands (seguimos yendo a saludarles y hemos de pasar varias veces para tener un hueco… )
– Ayer mucha concurrencia en la calle del Café Madrid … costó hoy llegar …
– hoy van las tarjetas de crédito en los restaurantes … (Thanks God)
– MEDICA Tech (IT, LAB, …) Forum : hoy con ciberseguridad, LDT, …
– Reencuentro con muchas caras conocidas, hoy internacionales (Elem, Monir, Ludger, Ronald, Carlos, Susana, Antonio, …)
– anunciada huelga de trenes … (nos ha fastidiado la visita a Colonia … )
– no llueve por la mañana … a ver si aguanta a la salida
mañana mas read more…

Hoy, 7 Abril, celebramos el Dia Mundial de la Salud #DíaMundialSalud #WorldHealthDay #WHO75 @SEEIC_Spain @SEIB_twit @SEISeSalud @AEIHorg @CEDifmbe @AAMI_connect @WHO_Europe @OMS_Spain @fenin_es @AEMPSgob @AEFI_es @tecno_med
El 7 de Abril celebramos el Día Mundial de la Salud – World Health Day, este año con el lema de la OMS «Mi salud, mi derecho»
Es una celebración mundial que reconoce la importante contribución de la sanidad en el bienestar de la población mundial.
El sector de la Tecnología Médico-Sanitaria se suma a esta fiesta y reivindica el acceso a la innovación y el suministro ininterrumpido de productos sanitarios.

Congreso SEEIC del 5-7 Junio 2024 «La tecnología al servicio del paciente» @SEEIC_spain con todas las novedades de la Ingeniería Clínica española

el 7 Abril celebramos el Dia Mundial de la Salud #DíaMundialSalud #WorldHealthDay #WHO75 @SEEIC_Spain @SEIB_twit @SEISeSalud @AEIHorg @CEDifmbe @AAMI_connect @WHO_Europe @OMS_Spain @fenin_es @AEMPSgob @AEFI_es @tecno_med
El 7 de Abril celebramos el Día Mundial de la Salud – World Health Day, este año con el lema de la OMS «Mi salud, mi derecho»
Es una celebración mundial que reconoce la importante contribución de la sanidad en el bienestar de la población mundial.
El sector de la Tecnología Médico-Sanitaria se suma a esta fiesta y reivindica el acceso a la innovación y el suministro ininterrumpido de productos sanitarios.

@AEFI_es de 8 a 9 mayo 2024 en Madrid – participa @tecno_med en Taller 6 (9 mayo 12h) «MARCADO CE MDSW con IA»
Como cada año tenemos una cita en el 42 SYMPOSIUM AEFI https://aefi2024.com/ este año el 8 y 9 de Mayo de 2024 en Madrid. Participamos en este magnifico encuentro de profesionales con el taller: «Marcado CE de aplicaciones informáticas médicas incluyendo Inteligencia Artificial» C.Murphy y X.Canals
Las nuevas aplicaciones IA, tanto MDR como IVDR, van a tener un efecto multiplicador de la capacidad asistencial de los profesionales sanitarios permitiendo abordar la asignatura pendiente de prevención y seguimiento de pacientes. Revisaremos en este taller como se obtiene el marcado CE de estas aplicaciones IA
No te pierdas este evento, esperamos poder saludarte allí.
Os iremos informando …

11-12 Jun 2024 «Jornadas de Enfermería Quirúrgica y Esterilización HURyC» by #UnidadCentralEsterilización Hospital Universitario Ramon y Cajal de @SaludMadrid con la participación de @Xcanals – @Tecno_med
La Unidad Central de Esterilización del Hospital Ramon y Cajal organiza las «Jornadas de Enfermería Quirúrgica y Esterilización HURyC» 2024. Tecno-med participa como ponentes en la misma.
12 Junio MESA 4 9h
Titulo: «Impacto del RD 192/2023 y el reglamento MDR en el Bloque quirúrgico y la CE»
Objetivo: La legislación de productos sanitarios esta en un proceso de cambio global para mejorar la seguridad del paciente impactando en la operación del Hospital y nos presenta nuevos desafíos y oportunidades: instrumentos quirúrgicos reutilizables con marcado CE con ON, etiquetado productos con UDI y esteriles con dos fechas, esterilización p. impresión 3D, fabricación in-house, validaciones, posible reprocesado productos un sólo uso, …
Ponente: Xavier Canals Riera. Euroingeniero. Director Tecno-med Ingenieros. Vicepresidente Sociedad Española Ing. Clínica SEEIC, miembro de SEDE, AAMI, RAPS.![]()

#SEDE Sociedad Española de Desinfección y Esterilización organiza su Congreso en Murcia el 6 y 7 junio 2024 con la participación de @3mhealthcare , @matachana_group , @elautoclave , …
La Sociedad Española de Desinfección y Esterilización (SEDE) organiza este año su Congreso donde se tratan las novedades. SEDE es el miembro español de la World Federation of Hospital Sterilization Sciences (WFHSS).
Si este tema te interesa hazte socio en https://sede.org.es/

Congreso ANCEI Asociación Nacional de Comités Eticos de Investigación Clínica 16-17 Mayo 2024 en Logroño by @ANCEIes con la participación de @xcanals
Congreso ANCEI – Asociación Nacional de Comités de Ética de la Investigación que tendrá lugar los días 16-17, 2024 de Mayo en Logroño con el lema : “10 años promoviendo la ética en la investigación”. Visita la web del congreso: https://www.congresoancei2024.com/ en este participa Xavier Canals (Director Tecno-med y miembro del CEIM del Hospital Clínic de Barcelona) en los talleres de productos sanitarios del 16 de mayo 14:30-16:30 REUNIÓN CON EL EXPERTO. TALLERES: 2. TALLER: Investigación con productos sanitarios: app, estudios combinados (medicamentos y productos sanitarios). ![]()

La @AEMPS se hace eco de la Consulta publica de la Comisión Europea sobre la Evaluación de Tecnologias Sanitarias – participa !!
La Comisión en su pagina web bajo el apartado «Díganos lo que piensa – Consultas públicas y comentarios» quiere conocer la opinión de las distintas partes interesadas sobre la legislación y las políticas actualmente en desarrollo. La evaluación de Tecnologias Sanitarias incluye a medicamentos y productos sanitarios.
Se pueden ver los comentarios publicados o enviar sus propios comentarios.

@infarma_es 19 a 21 Marzo 2024 Madrid
El encuentro del sector de farma donde siempre se pueden ver tendencias y novedades. Siempre interesante.
Regístrate ya en su web: https://www.infarma.es/

@RAPSorg «RAPS 2024 Euro Convergence» 6-8 Mayo 2024 Berlin
Como cada año no te pierdas este evento
https://www.raps.org/europe-2024/home
Algunas fotos de la pasada edición
Fantásticas las jornadas de RAPS https://www.raps.org/europe-2023/home , para ponernos al día con los grandes expertos europeos. En esta imagen la interesante mesa sobre el paciente con la participación de todos los actores incluida la EMA con el Dr Juan Garcia Burgos.
Nos reunimos allí el grupo de LNG Spain

Formación «2405 – IMPORTADORES Y DISTRIBUIDORES DE PROD.SANIT. según REQUISITOS MDR / IVDR y RD» – 18 Abr 2024 9h-14h
Ahora que nos acercamos al 26 de mayo de 2024 se vuelven mas relevantes los reglamentos MDR/IVDR y nuevo Real Decreto 192/2023 (RD IVD en borrador) que incrementan los requisitos a los importadores y distribuidores involucrados en el suministro, instalación, formación y mantenimiento de productos sanitarios. Ahora estamos en un periodo transitorio en el que importadores y distribuidores deben revisar el cumplimiento por parte del fabricante de los productos para su introducción en el mercado y liberación por el importador y comercialización por los distribuidores.
En esta formación, revisaremos las obligaciones de los importadores y distribuidores así como el impacto de los requisitos reglamentarios en toda la cadena de suministro. Tenemos la posibilidad de realizar mas actividades antes consideradas exclusivas de fabricante como reembalado y traducción de IFU según art.16 pero con requisito de sistema de calidad ISO 13485 certificado, también el registro del UDI-DI, el nuevo Registro de Comercialización de la AEMPS, …
No te pierdas esta jornada donde contaremos las novedades relativas a estas actividades según los nuevos reglamentos para estos periodos transitorios y como verificar el cumplimiento.
Fecha: 18 Abril 2024, 9h a 14h en webinar en directo y 25h en teleformación
Esperamos verte ahi … ![]()

Formación «2405 – IMPORTADORES Y DISTRIBUIDORES DE PROD.SANIT. según REQUISITOS MDR / IVDR y RD» – 18 Abr 2024 9h-14h
Los reglamentos MDR/IVDR y nuevo Real Decreto 192/2023 (RD IVD en borrador) refuerzan los requisitos a los importadores y distribuidores involucrados en el suministro, instalación, formación y mantenimiento de productos sanitarios. En esta formación, revisaremos las obligaciones de los importadores y distribuidores así como el impacto de los requisitos reglamentarios en toda la cadena de suministro. Tenemos la posibilidad de realizar mas actividades antes consideradas exclusivas de fabricante como reembalado y traducción de IFU según art.16 pero con requisito de sistema de calidad ISO 13485 certificado, también el registro del UDI-DI, el nuevo Registro de Comercialización de la AEMPS, …
No te pierdas esta jornada donde contaremos las novedades relativas a estas actividades según los nuevos reglamentos
Fecha: 18 Abril 2024, 9h a 14h en webinar en directo y 25h en teleformación
Esperamos verte ahi … ![]()

Formación «2404 – ISO 13485 y REQUISITOS de CALIDAD según MDR / IVDR y MDSAP» – 14 Mar 2024 9h-14h
Los reglamentos MDR e IVDR incluyen como requisito en su articulo 10 de un sistema de gestión de la calidad, siendo la norma armonizada con ambos reglamentos la EN ISO 13485:2016+AC:2018+A11:2021. En esta formación, focalizaremos en las obligaciones reglamentarias específicas incluyendo además de Europa las áreas reglamentarias de MDSAP y su impacto en los procesos, procedimientos y auditorÍas.
No te pierdas esta jornada donde contaremos las novedades sobre calidad en productos sanitarios
Fecha: 14 Marzo 2024, 9h a 14h en webinar en directo y 25h en teleformación
Esperamos verte ahi … ![]()
Intentamos resumir los cambios para productos sanitarios …

Formación «2405 – IMPORTADORES Y DISTRIBUIDORES DE PROD.SANIT. según REQUISITOS MDR / IVDR y RD» – 18 Abr 2024 9h-14h
Los reglamentos MDR/IVDR han reforzado los requisitos a los importadores y distribuidores involucrados en el suministro, instalación, formación y mantenimiento de productos sanitarios. En esta formación, revisaremos las obligaciones de los importadores y distribuidores así como el impacto de los requisitos reglamentarios en toda la cadena de suministro.
No te pierdas esta jornada donde contaremos las novedades relativas a estas actividades según los nuevos reglamentos
Fecha: 18 Abril 2024, 9h a 14h en webinar en directo y 25h en teleformación
Esperamos verte ahi … ![]()

Formación «2402 – COMERCIALIZACIÓN PRODUCTOS SANITARIOS USA – FDA» – 8 Feb 2024 9h-14h
El principal destino de exportación europeo de productos sanitarios es USA. Por lo que revisaremos como a partir de la documentación técnica y sistema de calidad para el Marcado CE podemos adaptarla para obtener la autorización para la comercialización en USA presentando un 510K y el cumplimiento de la 21 CFR 820 Quality System Regulations.
Con cambios muy relevantes en este ultimo año que van desde guías nuevas de evaluación de productos via 510k, guías de ensayos preclinicos y clínicos, guías QSUB, guías esterilización, guías software, …
No te pierdas esta jornada donde contaremos las novedades y como obtener el clearance de FDA y comercializar tus productos sanitarios en USA.
Fecha: 8 Febrero 2024, 9h a 14h en webinar en directo y 25h en teleformación
Esperamos verte ahi … ![]()
Intentamos resumir los cambios para productos sanitarios …

Formación AI MDSW ahora online «2401T – SOFTWARE MÉDICO e INTELIGENCIA ARTIFICIAL» con las novedades normativas y de guías
Este diciembre se aprobó el texto de la AIA Artificial Intelligence Act que incluye al software médico con IA como crítico y también diversas normas asociadas a las aplicaciones informáticas médicas que incluyen IA, como la ISO/IEC 42001 sistema gestión AI, la BSI AAMI 34971 riesgos AI, … por la ISO/IEC JTC 1/SC42
Y también tenemos las actualizaciones de las guías de documentación técnica de software de Team-NB, de FDA y de IG-NB …
Las aplicaciones de software médico y las aplicaciones de software que incluyen inteligencia artificial han provocado un avance disruptivo en los diagnósticos y tratamientos sanitarios. En esta formación revisamos sus requisitos como producto sanitario incluyendo la nueva normativa asociada a interoperabilidad, ciberseguridad, integridad de datos y protección de datos.
Disponible ahora la versión grabada de la realizada en fecha: 18 Enero 2024
![]()
Felices Navidades !!! os deseamos desde @tecno_med
Formación «2401 – SOFTWARE MÉDICO e INTELIGENCIA ARTIFICIAL» – 18 En 2024 9h-14h con las novedades normativas y de guías
Este diciembre se aprobó el texto de la AIA Artificial Intelligence Act que incluye al software médico con IA como crítico y también diversas normas asociadas a las aplicaciones informáticas médicas que incluyen IA, como la ISO/IEC 42001 sistema gestión AI, la BSI AAMI 34971 riesgos AI, … por la ISO/IEC JTC 1/SC42
Y también tenemos las actualizaciones de las guías de documentación técnica de software de Team-NB, de FDA y de IG-NB …
Las aplicaciones de software médico y las aplicaciones de software que incluyen inteligencia artificial han provocado un avance disruptivo en los diagnósticos y tratamientos sanitarios. En esta formación revisamos sus requisitos como producto sanitario incluyendo la nueva normativa asociada a interoperabilidad, ciberseguridad, integridad de datos y protección de datos.
Fecha: 18 Enero 2024, 9h a 14h en webinar en directo y 25h en teleformación
Esperamos verte ahi … ![]()

Black friday «BF-23» hasta el 30 de noviembre 40% dto en formación online @Tecno_Sanitaria

Formación «2309 – MARCADO CE MDR» – 12 Dic 2023 y «2310 – MARCADO CE IVDR» – 14 Dic 2023 9h-14h
El Reglamento de Productos Sanitarios MDR se aplica desde 26/05/2021 quedando derogadas las Directivas, es un cambio muy relevante que marcará un antes y un después en Europa.
En esta formación nos hemos puesto de objetivos contestar a las siguientes cuestiones:
– ¿Cuánto me queda para los legacy? Que debo hacer?
– Requisitos MDR? UDI? EUDAMED? RD 192/2023?
– Nuevos productos: ¿freno a la innovación?
– Productos sanitarios huérfanos? Encuestas …
lo analizaremos viendo sus requisitos: – Mercado regulado Evaluación de conformidad pre-market; – Clasificación según riesgo de productos; – Documentación Técnica; – Sistema de Gestión de Calidad y registros/licencia;
veremos un CASO PRACTICO y como siempre queremos compartir los problemas y lecciones aprendidas con vosotros
Fecha: 12 Diciembre 2023, 9h a 14h en webinar en directo y 25h en teleformación hasta el día 31 de diciembre de 2023
Esperamos verte ahi … ![]()
El Reglamento de Productos Sanitarios para diagnóstico in vitro IVDR se aplica desde 26/05/2022 quedando derogadas las Directivas, es un cambio muy relevante que marcará un antes y un después en Europa.
En esta formación nos hemos puesto de objetivos contestar a las siguientes cuestiones:
– ¿Cuánto me queda para los legacy? Que debo hacer?
– Requisitos IVDR? UDI? EUDAMED? RD IVD?
– Nuevos productos: ¿freno a la innovación?
– Productos sanitarios huérfanos? Encuestas …
lo analizaremos viendo sus requisitos: – Mercado regulado Evaluación de conformidad pre-market; – Clasificación según riesgo de productos; – Documentación Técnica; – Sistema de Gestión de Calidad y registros/licencia;
veremos un CASO PRACTICO y como siempre queremos compartir los problemas y lecciones aprendidas con vosotros
Fecha: 14 Diciembre 2023, 9h a 14h en webinar en directo y 25h en teleformación hasta el día 31 de diciembre de 2023
Esperamos verte ahi … ![]()
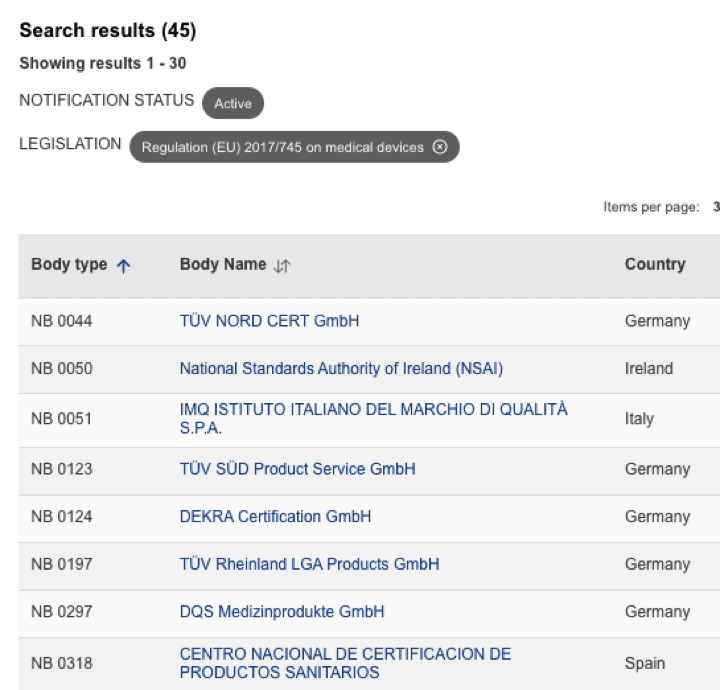
Organismos Notificados MDR (45): TUV SUD DANMARK (Dinamarca) ON num. 2443 nuevo Organismo Notificado. Enhorabuena !!!
Nueva designación del Organismo Notificado:
TÜV SÜD Danmark
Address Strandvejen 125; 2900 Hellerup; Denmark
Email: info.dk@tuvsud.com
Website https://www.tuvsud.com/en-gb/country/denmark
puedes ver la lista siempre actualizada en la base de datos NANDO:
https://webgate.ec.europa.eu/single-market-compliance-space/#/notified-bodies/notified-body-list?filter=legislationId:34,bodyTypeId:3,notificationStatusId:1
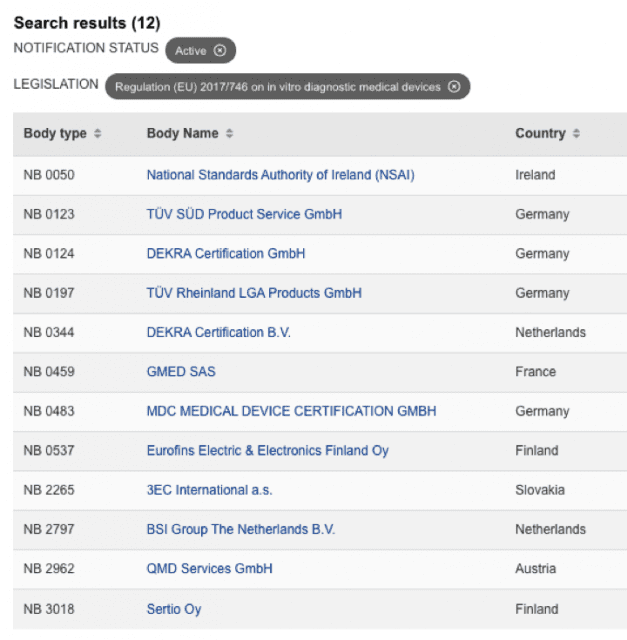
y para IVDR …
lista actualizada IVDR en:
https://webgate.ec.europa.eu/single-market-compliance-space/#/notified-bodies/notified-body-list?filter=legislationId:35,bodyTypeId:3,notificationStatusId:1

Nueva edición de la norma EN ISO 20196:2024 productos sanitarios ivd. Estudios del funcionamiento clínico con muestras de seres humanos – Buenas practicas de estudio
La esperada norma para los estudios de funcionamiento clínico según anexo XIII.2 de IVDR y que es la analoga a la ISO 14155 para producto sanitario general. Descargate la vista preliminar de la misma. Incluye anexo ZA con IVDR referenciando a los requisitos generales de seguridad y funcionamiento que da conformidad: 1, 2, 3, 4, 5, 8, 10, 11, 13, 15, 16, 17, 18 y 19 e incluye el anexo Z
Adhiérete a nuestro contrato de vigilancia tecnológica y estarás al día de las novedades normativas y reglamentarias. No más sorpresas, entérate antes de que sea una no conformidad. https://www.meddev.biz/b2c/producto/WEB105/1/vigilancia-tecnologica-productos-sanitarios

Publicación de @EU_Health Comisión Europea de la revisión 1 de los requisitos relativos al idioma según MDR / IVDR
La Dirección General de Salud y Seguridad Alimentaria (DG SANTE) de la Comisión Europea actualiza las tablas de requisitos de idioma en el etiquetado e instrucciones de uso de productos sanitarios relativos a cada país que puede regularlo según indica MDR e IVDR. Ademas incluye las interfases de usuario para las aplicaciones informáticas.
Descárgatelas aqui TABLA MDR , TABLA IVDR
La Comisión Europea publica una nueva actualización del NB Survey de adaptación a MDR/IVDR

@MedtechEurope ( @FENIN_es ) publica el informe «perspective on the final AI Act»

MDCG: publicada nueva MDCG 2024-3 guia contenido CIP Plan de Investigación Clínica

Estado #AIA Artificial Intelligence Act – adoptada en la sesión plenaria del 13 de marzo de 2024 por el Parlamento
El Reglamento, acordado en las negociaciones con los Estados miembros en diciembre de 2023, fue respaldado por la Eurocámara con 523 votos a favor, 46 en contra y 49 abstenciones.
Su objetivo es proteger los derechos fundamentales, la democracia, el Estado de derecho y la sostenibilidad medioambiental frente a la IA que entraña un alto riesgo, impulsando al mismo tiempo la innovación y erigiendo a Europa en líder del sector. El Reglamento fija una serie de obligaciones para la IA en función de sus riesgos potenciales y su nivel de impacto.
Según el articulo 6 se consideran de alto riesgo los sistemas de IA que están cubiertos en la legislación indicada en anexo II (incluye MDR e IVDR)
Próximos pasos
El Reglamento aún está sujeto a una última comprobación jurídica-lingüística. Su aprobación definitiva (mediante el llamado procedimiento de corrección de errores) está prevista para antes del final de la legislatura. La ley también debe ser adoptada formalmente por el Consejo. Entrará en vigor veinte días después de su publicación en el Diario Oficial y será de plena aplicación veinticuatro meses después de su entrada en vigor, con excepción de: las prohibiciones de prácticas (se aplicarán seis meses después de la fecha de entrada en vigor); los códigos de buenas prácticas (nueve meses después); las normas sobre la IA de uso general, incluida la gobernanza (doce meses después), y las obligaciones para los sistemas de alto riesgo (treinta y seis meses después).
Os dejamos aqui el texto en español. Buena lectura
seguiremos informando ..

Nueva publicacion de la FDA para AI MDSW y su tratamiento por sus departamentos @FDAdeviceInfo

Publicada nueva lista de normas armonizadas con IVDR (13) – Decisión de Ejecución (UE) 2024/817
Se actualiza la lista de normas armonizadas ahora tenemos 13 read more…